Mitochondrial Dysfunction and Metabolic Reprogramming in Multiple Sclerosis
Multiple Sclerosis (MS) is a complex immune-mediated neurodegenerative disease that predominantly affects adults, leading to the demyelination of nerves. The disease is characterized by periods of relapses and remissions in its most common form, relapsing-remitting MS (RRMS), and continues to pose significant diagnostic and therapeutic challenges. While the genetic and environmental factors contributing to MS are well-documented, recent research is shedding light on the crucial role of mitochondrial dysfunction and metabolic reprogramming in the pathogenesis of MS, particularly within peripheral immune cells.
The Mitochondrial Connection
Mitochondria are known as the powerhouses of the cell, responsible for producing energy through oxidative phosphorylation. However, their role in MS goes beyond mere energy production. Studies have identified significant mitochondrial abnormalities in the immune cells of MS patients, including altered mitochondrial DNA (mtDNA) levels, impaired mitochondrial respiration, and increased production of reactive oxygen species (ROS). These dysfunctions contribute to the aberrant immune responses observed in MS, where immune cells mistakenly attack the myelin sheath of nerve fibers.
Immune Cells and Mitochondrial Dysfunction
T Cells: T cells are central players in the immune response in MS. Mitochondrial dysfunction in T cells has been linked to altered glycolysis and oxidative phosphorylation (OXPHOS), leading to increased ROS production. This metabolic reprogramming supports the proliferation and activation of autoreactive T cells, particularly Th17 cells, which are known to drive inflammation in MS. Therapeutic interventions, such as treatment with interferon-beta, have been shown to restore mitochondrial function and reduce T cell-mediated damage.
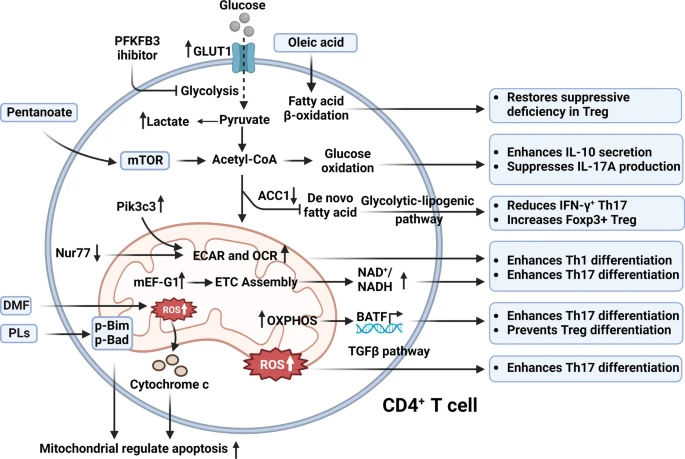
Mitochondrial metabolism in MS CD4+ T cells involves enhanced glucose uptake, fatty acid oxidation, and oxidative phosphorylation, influencing the balance between Th17 differentiation and Treg function, with key metabolic pathways modulating immune responses and apoptosis through mitochondrial ROS and OXPHOS activity. (Wang, P. F., et. al. (2024))
B Cells: B cells, which are responsible for antibody production, also exhibit mitochondrial abnormalities in MS. Increased glycolysis and cholesterol metabolism in B cells from MS patients suggest a shift towards a pro-inflammatory state. The mitochondrial dysfunction in B cells contributes to their enhanced antigen-presenting capabilities and promotes the production of autoantibodies that target the CNS.
Monocytes and Macrophages: Monocytes, upon entering the CNS, can differentiate into macrophages, which are key mediators of inflammation and tissue damage in MS. These cells exhibit increased glycolytic activity and ROS production, driven by mitochondrial dysfunction. This metabolic shift not only fuels the inflammatory response but also impairs the clearance of myelin debris, exacerbating neurodegeneration.
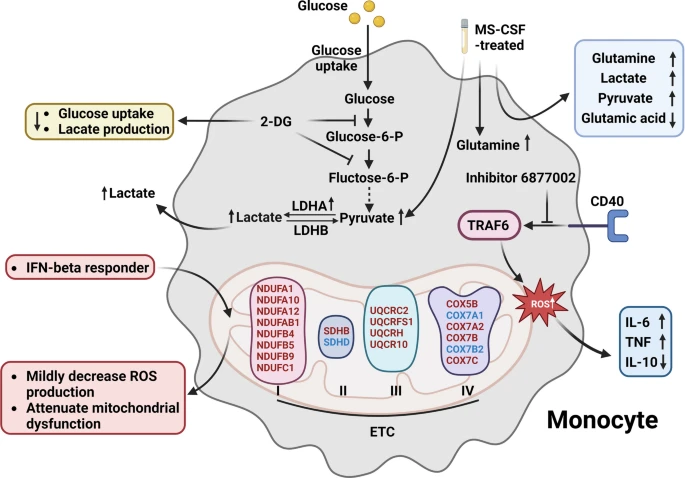
Mitochondrial dysfunction in MS monocytes, characterized by increased lactate production via LDHA, inhibition of glycolysis by 2-DG, altered metabolic responses to CSF exposure, and modulation of oxidative stress and inflammatory cytokine production by the small molecule inhibitor 6877002 and IFN-β treatment. (Wang, P. F., et. al. (2024))
Dendritic Cells (DCs): DCs are crucial for initiating immune responses by presenting antigens to T cells. In MS, mitochondrial dysfunction in DCs leads to altered antigen processing and presentation, promoting the activation of autoreactive T cells. The metabolic reprogramming of DCs, including increased ROS production, further amplifies the autoimmune response.
Therapeutic Implications
Understanding the role of mitochondrial dysfunction in MS opens up new avenues for therapeutic interventions. Targeting the metabolic pathways involved in immune cell activation could help in modulating the immune response and slowing disease progression. For instance, dimethyl fumarate (DMF) has been shown to reduce ROS production and restore mitochondrial function in T cells and macrophages, thereby limiting inflammation and neurodegeneration.
Conclusion
The intricate relationship between mitochondrial dysfunction and immune cell metabolism in MS underscores the complexity of the disease and highlights potential targets for novel therapies. As research continues to unravel the mitochondrial pathways involved in MS, there is hope for developing more effective treatments that not only manage symptoms but also address the underlying metabolic dysregulation driving the disease.
This review from Wang et al. provides a comprehensive overview of the current understanding of mitochondrial dysfunction in MS, emphasizing the need for further research into the metabolic aspects of immune regulation in this devastating disease.
References:
Wang, P. F., Jiang, F., Zeng, Q. M., Yin, W. F., Hu, Y. Z., Li, Q., & Hu, Z. L. (2024). Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis. Journal of neuroinflammation, 21(1), 28.

